💉SYNTHESIS OF SALICYLIC ACID AND ACETYLSALICYLIC ACID FROM POLYSTYRENE RESIDUE💉
Abstract
In this project we have been able to synthesize salicylic acid and acetylsalicylic acid from polystyrene residues. The yield of the synthetic route from polystyrene to acetylsalicylic acid was just 8,2%. However, we know of various aspects of the process that could be improved to increase the overall yield.
1 Introducction
This work is a continuation of the one previously presented in 2022 to the “Investigar en ciencia” awards of the UEX, entitled “Aprovechamiento de residuos plásticos transformados en combustible mediante pirólisis y su impacto climático” (Use of plastic waste transformed into fuel by pyrolysis and its climatic impact).
In this work we have carried out the synthesis of salicylic acid and acetylsalicylic acid from expanded polystyrene. Expanded polystyrene is a polymer widely used in countless everyday products, although as with most plastics, these products are single-use. On the other hand, salicylic acid is a compound of great health importance, since it is the precursor of drugs such as mesalazine, used as an intestinal anti-inflammatory; bismuth subsalicylate, used to treat heartburn; or acetylsalicylic acid, commonly known as aspirin, which has analgesic and anticoagulant properties. It is the latter that we have synthesized in this work.
2 Synthesis
The synthetic route (Scheme 1) from polystyrene to acetylsalicylic acid can be divided into four stages:
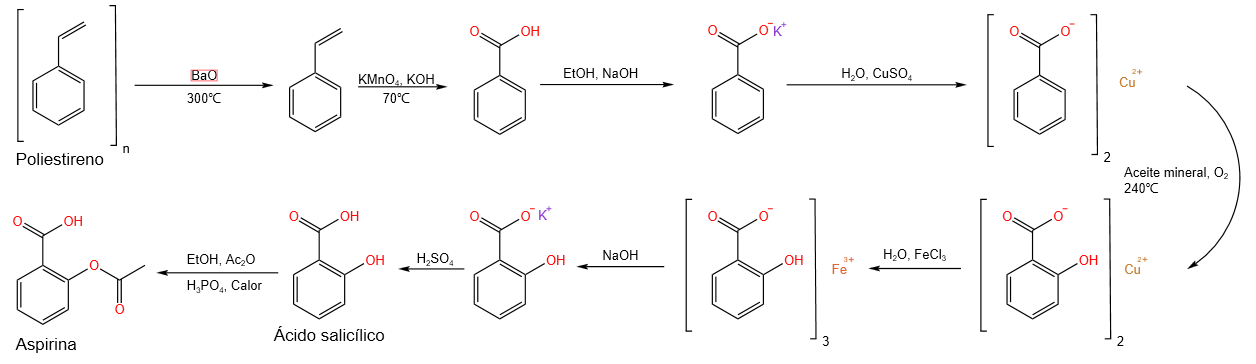
2.1 Depolymerization
The depolymerization reaction is shown in Scheme 2. A conventional distillation apparatus heated with a heating mantle was used as the pyrolysis reactor. 113.2 g of polystyrene and 1.28 g of BaO, which acts as a catalyst, were introduced into the distillation flask. The reaction was terminated when liquid stopped collecting in the collecting flask 102 g of a yellowish oily liquid were obtained, which was subsequently distilled, collecting 72.4 g of the fraction obtained at 145º, the boiling point corresponding to styrene (Image 1). The yield of the reaction was 63.9 %.
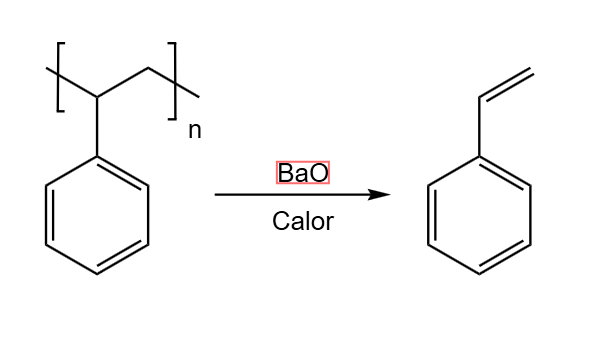

2.2 Oxidation
The oxidation of styrene to benzoic acid, shown in Scheme 3, was carried out with potassium permanganate in an aqueous basic medium. In a round flask with three openings, placing a water-cooled reflux condenser in the middle one, and a thermometer and an addition funnel at the sides.
To begin with, 5 ml of a 20% KOH solution in water together with 45 ml of distilled water and 4.26 g of styrene were added to the flask. The mixture was allowed to warm up to 70°. Once this temperature was reached, a solution previously heated to 70ºC formed by 150 ml of distilled water and 13 g of potassium permanganate was introduced into the addition funnel. The addition of the permanganate was carried out for 15 minutes to prevent the reaction from occurring too fast, then, the mixture was left at 70ºC for 90 minutes until the violet color of the permanganate was no longer visible.
To extract the benzoic acid, which is in solution in the form of potassium benzoate, the contents of the flask were first filtered, discarding the solid manganese dioxide (Image 2). To the liquid fraction was first added an arbitrary amount of sodium nitrite and then 45 ml of 1M sulfuric acid, thus dissolving any remaining manganese dioxide and precipitating the benzoic acid, which was subsequently filtered. Obtaining 3.81 g of dry product, giving a yield of 76% (Image 3).
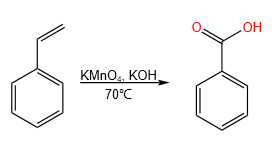


2.3 Thermal rearrangement
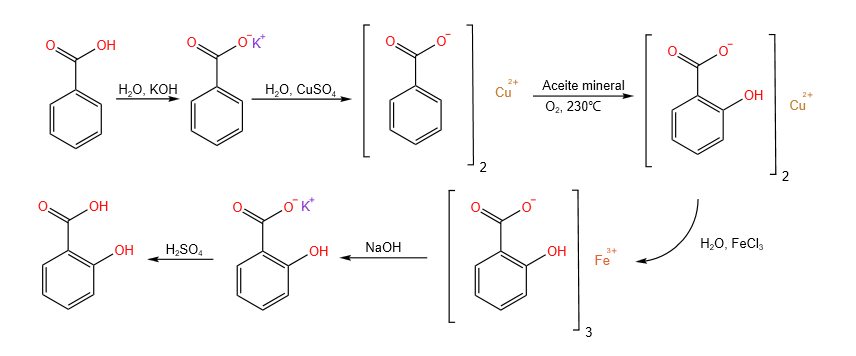
This reaction consists of the thermal rearrangement of copper benzoate in the presence of oxygen, giving rise to copper salicylate2 (Scheme 4).
To convert benzoic acid to copper benzoate, benzoic acid was first reacted with potassium hydroxide, forming potassium benzoate, which will subsequently form the desired copper benzoate upon reaction with copper(II) sulfate.
The reaction was carried out in a round flask with three openings, placing in the central one a reflux condenser cooled with water and in the lateral ones a thermometer and a tube connected to an air pump with a flow rate of 2 L/min.
In the flask 150 ml of mineral oil and 3.5 grams of copper benzoate were introduced (Figure 4). The reaction was allowed to heat until it reached 240°C, at which time the air pump was turned on for 50 min.
The mixture was allowed to cool to room temperature and this process was repeated two more times, performing a total of three cycles.
Once the reaction was completed, the solid residues were separated from the mineral oil by decanting. Subsequently, the solid residues were washed with a solution of iron trichloride in water, thus extracting the synthesized salicylic acid, since it forms a complex with the iron ions, with an intense purple color and soluble in water (Image 5). This iron(III) salicylate solution was reacted with KOH, precipitating iron hydroxide and leaving dissolved potassium salicylate. The mixture was then filtered, separating the iron from the acid, which was later reacted with sulfuric acid, precipitating salicylic acid. Once the acid was filtered, it was left to dry for 24 hours, obtaining 1.03 g, which gives an efficiency of 23.3% (Image 6).


2.4 Acetylation of salicylic acid.
Acetylsalicylic acid, commonly known as aspirin, is used as an analgesic, anti-inflammatory and antipyretic. For its synthesis, the 1.03 g of salicylic acid, 3 ml of acetic anhydride and 5 drops of concentrated sulfuric acid were added to a 50 ml erlenmeyer flask (Scheme 5). The flask was placed in a beaker with water, which was heated until it began to boil, at which time the heat was removed. After leaving the flask 10 minutes in the hot water, 5 ml of distilled water was added to decompose any remaining acetic anhydride, then, the flask was placed in a bath with ice to cool it and 20 ml of distilled water was added. After a few minutes, the contents of the flask were filtered and the acetylsalicylic acid was left to dry for 24 hours, obtaining 0.89 grams of product, giving an efficiency of 72.3% (Image 6).

3 Results and discussion.
3.1 Sample analysis.
Once the synthesis was completed, thin layer chromatography was carried out to verify that the acetylsalicylic acid had been synthesized (Image 7).
A mixture of 50% chloroform and 50% cyclohexane by volume was used as eluent. A solution consisting of 1.5 g KMnO4, 10 g K2CO3 and 1.25 ml of 10% NaOH in 200 ml of distilled water was used as a stain.
From left to right the dots represent: commercial salicylic acid, the synthesized salicylic acid, and the synthesized acetylsalicylic acid. From this test we can conclude that we have been able to correctly synthesize salicylic acid. We can also state with a fair degree of certainty that acetylsalicylic acid has been synthesized, since if the acetylation reaction had not occurred, the third mark would be at the same level as the others.

3.1 Sample analysis.
Once the synthesis was completed, thin layer chromatography was carried out to verify that the acetylsalicylic acid had been synthesized (Image 7).
A mixture of 50% chloroform and 50% cyclohexane by volume was used as eluent. A solution consisting of 1.5 g KMnO4, 10 g K2CO3 and 1.25 ml of 10% NaOH in 200 ml of distilled water was used as a stain.
From left to right the dots represent: commercial salicylic acid, the synthesized salicylic acid, and the synthesized acetylsalicylic acid. From this test we can conclude that we have been able to correctly synthesize salicylic acid. We can also state with a fair degree of certainty that acetylsalicylic acid has been synthesized, since if the acetylation reaction had not occurred, the third mark would be at the same level as the others.
3.2 Possible improvements and discussion of yields.
3.2.1 Oxidation
We believe that the efficiency of this reaction could have been higher, because the styrene used was partially polymerized, due to the long time it had been stored (2 months). There are several solutions to this problem, such as synthesizing the styrene at the time, or inhibiting its polymerization by storing it at low temperature.
3.2.2 Thermal rearrangement
The efficiency we obtained in the thermal rearrangement was considerably lower than that reported in the available literature2 , ours being 23.3%, against 40.3% in the literature for 3 cycles. We believe that this may be due to the fact that during the reaction, the magnetic stirrer of the heating plate stopped working, although we do not know at what point it failed. If it had failed between the end of the second and the beginning of the third cycle, the efficiency obtained would be much closer to that expected for two cycles (24.7%).
4 Conclusion
From this work we can conclude that it is possible to use polystyrene as a raw material for the synthesis of organic compounds of major interest and importance, without the need for specialized equipment.
about me
monster addict with an irrational love for science (mostly biochem, ochem and electronics) and cat enthusiast born in 2006 in a spanish village lost from the hand of god 
Download PDF (spanish)
about this article
This project started in June 2022 as my second research project in highschool, and endeed around Feb 2024. I was awarded first place in a research contest hosted by UEX aimed towards highschoolers (nothing serious, there weren't a lot of projects presented either)